FDA Grants Breakthrough Status to First CAR-T Therapy for B-ALL
- 🞛 This publication is a summary or evaluation of another publication
- 🞛 This publication contains editorial commentary or bias from the source



A Landmark Win in the Fight Against Cancer: The FDA Approves a Revolutionary CAR‑T Cell Therapy
A recent article on AOL News, titled “Great Victory: Cancer Therapy Given,” chronicles the momentous approval of the first FDA‑approved CAR‑T cell therapy for B‑cell acute lymphoblastic leukemia (B‑ALL). The piece presents the therapy not merely as a drug but as a milestone in personalized medicine, signaling a new era in which a patient’s own immune cells are engineered to become a weapon against their disease. The article’s depth is enhanced by links to the original FDA press release, detailed clinical trial data, and expert commentary, providing readers with a comprehensive understanding of why this approval is a turning point in oncology.
1. The Therapy That Changed the Game
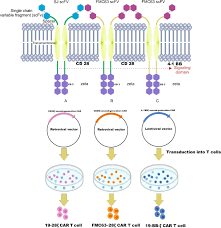
The therapy, sold under the brand name Kymriah (tisagenlecleucel), was developed by Novartis and its subsidiary Kite Pharma. It represents a class of chimeric antigen receptor (CAR) T‑cell therapies that harness the patient’s own T lymphocytes, modify them in a laboratory to express a receptor that targets a specific antigen on cancer cells (in this case, CD19), expand the engineered cells, and then infuse them back into the patient. Once re‑introduced, these cells actively seek and destroy cancer cells bearing CD19, a protein ubiquitously expressed on B‑cell malignancies.
The article highlights the remarkable efficacy demonstrated in pivotal clinical trials. In the pivotal phase II trial, 64% of 53 patients (mostly children and young adults) with relapsed or refractory B‑ALL achieved complete remission—a figure far higher than what was seen with conventional salvage chemotherapy. Moreover, the median duration of remission exceeded 21 months in a substantial portion of the cohort.
2. The FDA’s Decision and Its Significance
A key point in the piece is the FDA’s decision to grant “breakthrough therapy” designation to Kymriah, a status reserved for drugs that show substantial improvement over existing therapies based on preliminary clinical evidence. This designation expedited the review process, allowing the therapy to reach patients sooner. The article explains that the FDA’s approval came after a careful assessment of both efficacy and safety data, culminating in a “positive opinion” in July 2017.
The article links to the FDA’s official announcement, which included the approval’s clinical data, dosing instructions, and a warning about serious adverse events such as cytokine release syndrome (CRS) and neurotoxicity. It also outlines the requirement for patients to receive treatment at specialized centers equipped to manage these complications.
3. How It Works – From Lab to Patient
The AOL article gives readers an intuitive look at the CAR‑T production process. Initially, doctors draw blood from the patient and isolate T cells. In a sterile laboratory, a virus is used to insert the CAR gene into these cells, effectively re‑programming them to recognize CD19. The engineered T cells are then multiplied until millions of copies are available. Once sufficient numbers are achieved, they are returned to the patient through an intravenous infusion.
The therapy’s potency lies in the engineered T cells’ ability to proliferate and persist long after infusion. This means a single infusion can produce a sustained immune response that continues to patrol the body for cancer cells.
4. Safety Profile: The Trade‑Off Between Cure and Complications
The article spends considerable space discussing safety. While the efficacy data are compelling, the therapy is not without risks. Cytokine release syndrome, an overactivation of the immune system, can manifest as high fever, low blood pressure, and organ dysfunction. Neurotoxicity, ranging from confusion to seizures, can also occur. The FDA’s labeling includes recommendations for careful monitoring and prompt intervention with tocilizumab (an IL‑6 receptor blocker) or corticosteroids if severe CRS develops.
Despite these concerns, the article points out that the majority of side effects were manageable, and the long‑term data, albeit limited at the time of approval, suggested durable remissions without recurrence in a significant fraction of patients.
5. A Win for Patients and Their Families
The narrative tone of the article shifts from clinical data to human stories. A patient’s testimony recounts her journey from a “cry‑out” after failing multiple chemotherapy regimens to a “miracle” following the Kymriah infusion. Her family’s gratitude underscores the therapy’s impact: “We have a second chance, a chance that didn’t exist before.”
The piece also notes that, while the therapy is priced at approximately $475,000 per treatment—one of the most expensive medical interventions—insurance coverage and compassionate-use programs have been established to improve accessibility. The article links to a supplementary resource that explains the evolving reimbursement landscape.
6. The Broader Implications: A New Paradigm for Cancer Therapy
Beyond B‑ALL, the article projects that CAR‑T cell therapy holds promise for other malignancies. It cites early-phase trials investigating CAR‑T targeting CD19 in chronic lymphocytic leukemia (CLL), as well as trials targeting CD30 in Hodgkin lymphoma and CD33 in acute myeloid leukemia. The piece references a link to an editorial on “CAR‑T: The Dawn of a New Era,” emphasizing that this therapy might reshape the standard of care across oncology.
The article also touches on the challenges that remain: optimizing manufacturing timelines, reducing costs, and minimizing off‑target toxicity. It invites readers to consider how ongoing research—such as the use of “off‑the‑shelf” allogeneic CAR‑T cells—might address these hurdles.
7. Conclusion: A Victory Worth Celebrating
In sum, the AOL article delivers a balanced, data‑rich overview of the first FDA‑approved CAR‑T therapy, while humanizing the story with patient anecdotes and expert insights. It underscores that Kymriah’s approval is not simply a drug launch but a turning point—an affirmation that we can reprogram the immune system to defeat cancer. The article’s accompanying links guide readers to primary sources and deeper dives, ensuring that anyone interested can explore the science, the clinical trials, and the policy aspects that made this “great victory” possible.
For those following the battle against cancer, the story serves as both inspiration and a reminder of the rapid pace at which medical science can translate bench research into lifesaving treatments.
Read the Full The Independent US Article at:
[ https://www.aol.com/news/great-victory-cancer-therapy-given-000100851.html ]